Context analysis
In recent years there has been a growing trend in large engineering companies specializing in the industrial production sector, and in particular pharmaceutical production, to include a specialized figure in their staff, called Process Architect or Process Architect in the Italian sense, capable of guaranteeing an integrated design between the various disciplines involved in the project.
Pharmaceutical factories must meet architectural criteria but also respond to complex technological needs, with particular attention to the integration between the various categories of work, such as process engineering, mechanical, electrical, and hydraulic engineering (MEP), without forgetting compliance to GMP (Good Manufacturing Practices) regulations.
Architects are generally trained to be integrators, organizers, and collaborators. Process Architects play a key role as three-dimensional thinking building designers who have a global vision of all disciplines and can connect the various actors involved in a project.
Project setup and the role of the Process Architect
Among the main activities carried out by a Process Architect, there is the definition of the Layout, where it is essential to address the integration of the equipment into it from the early stages of the design and to do it correctly the first time to avoid extra costs due to activities of redesign.
Based on the selected equipment, operating heights, accessibility for maintenance activities, flows and delivery logistics are addressed.
Initial design is typically based on generic machine models or, if parameters are unknown, worst case scenario assumptions are made.
With the aim of optimizing times and starting with an optimal project setup, it is important:
- Be aligned with the company’s business objectives.
- Share and determine the flow of personnel and materials to promote a common understanding of the layout.
- Simplify and define standard operating procedures such as hand washing or sanitizing and the use of personal protective equipment.
- Review critical sterility issues with all stakeholders, including the HSE.
- Provide an adequate number of Airlocks with accurate information on current required GMP (cGMP) zone classifications, Biocontainment and required pressurization.
- Choose and decide on the management of Airlocks and interlocks.
- Be careful of some design criteria, such as the ergonomic study of the machines and the degree of product handling, as well as the biological and toxicity levels for dangerous products such as flammable corrosive substances.
- Have a common understanding of GMP requirements.
- Identify, agree, and incorporate segregation between clean and dirty areas into the layout to avoid hindering the flow of products, materials, personnel and waste.
In addition to the important aspects to consider in the early design phases listed above, there are other challenges to face when the Process Architect is faced with renovation projects of existing production plants, where unforeseen events are inevitable and a rapid “problem solving” attitude is often required.
These may include negative pressure zones, erection of temporary partitions, construction of operational facilities, decontamination of spaces, dust management and clean waste removal.
Let’s start with the design
During design there are elements of design, functionality (flow and adjacencies) and space (environment and human scale) that must be balanced with respect to the reference regulations.
Starting with an understanding of the company vision, questions asked at the beginning of the project may include:
Which products are to be manufactured and which are the target commercial markets? Single or multiple products? What products are offered? Confinement levels, toxicity, etc.? Have regulatory requirements been met to sell the product(s) in the proposed markets?
Once products and markets are identified, regulatory guidelines, such as good manufacturing practices (GMP), building code regulations, local biosafety requirements, and company facility guidelines, including health standards, safety and environment (HSE), only then all these elements are incorporated into the project design.
Main project requirements
The layout of a pharmaceutical manufacturing plant it must be developed around the needs of the structure, and to precisely identify the needs, the “must have” objectives must be separated from the non-strategic objectives.
This classification of needs is often takes a long time, since each department needs to rethink what is truly necessary for their business versus those items that are only desirable, but not essential to operations.
Specifically, for example, regarding the definition of the layout of the structure, it must be an integrated project that meets the following requirements:
- Compliance with GMP
- User Requirements
- Equipment layout and equipment requirements
- Personnel and material flows (product, component, and raw material)
- Operational access requirements
- Maintenance access requirements
- Cost considerations in layout design
Below are the main characteristics of some of these requirements:
User Requirements
The designer must first understand the requirements of the product and the process, precisely clarifying the number and dimensions of the rooms that will be required, the relationships between the groups of rooms, the finishes, the equipment, the furniture suitable for carrying out the various functions.
Environmental conditions can include temperature, humidity, air movement, sound insulation, etc.
Equipment Layout and equipment requirements
- It defines all areas that can influence the operations necessary for production, as well as the relationships and flows between them.
- Ensuring the correct flow of materials and personnel is the main objective.
- It can be developed once the process is known.
- They are also called logic diagrams.
- It derives from the program and the sizing needs of the equipment.
- Blocks are developed to indicate the size of the equipment.
- Room groups are assembled based on necessary adjacencies and process requirements.
Dressing requirements
The dressing rooms play a fundamental role in the layout of the structure.
Locker rooms:
Two degrees (levels) of changing rooms:
- Low (standard)
- From normal clothes (civilian clothes) to factory (clean clothes).
- High
- From clean clothes to full coverage coveralls.
The project phases and the importance of involving the Process Architect from the beginning
A front-end design process is based on the axiom of “everyone engaged early” and it is extremely important not to skip any design level.
It is an integrative interdisciplinary effort that allows all stakeholders, including the Process Architect, to share information and collaborate to achieve common goals.
Involving the Process Architect at the beginning of the project allows the company itself to act as a consultant regarding risks and operability issues, “what-if” situations, concerns, and adherence to GMP procedures.
It is important to invest in a strong Conceptual Design which essentially leads to the following advantages:
- Low initial costs
- May include Feasibility study
- Advance clarification of the main project themes
- Stronger decision-making tool
- Possibility to develop alternatives/brainstorming
- Customer orientation
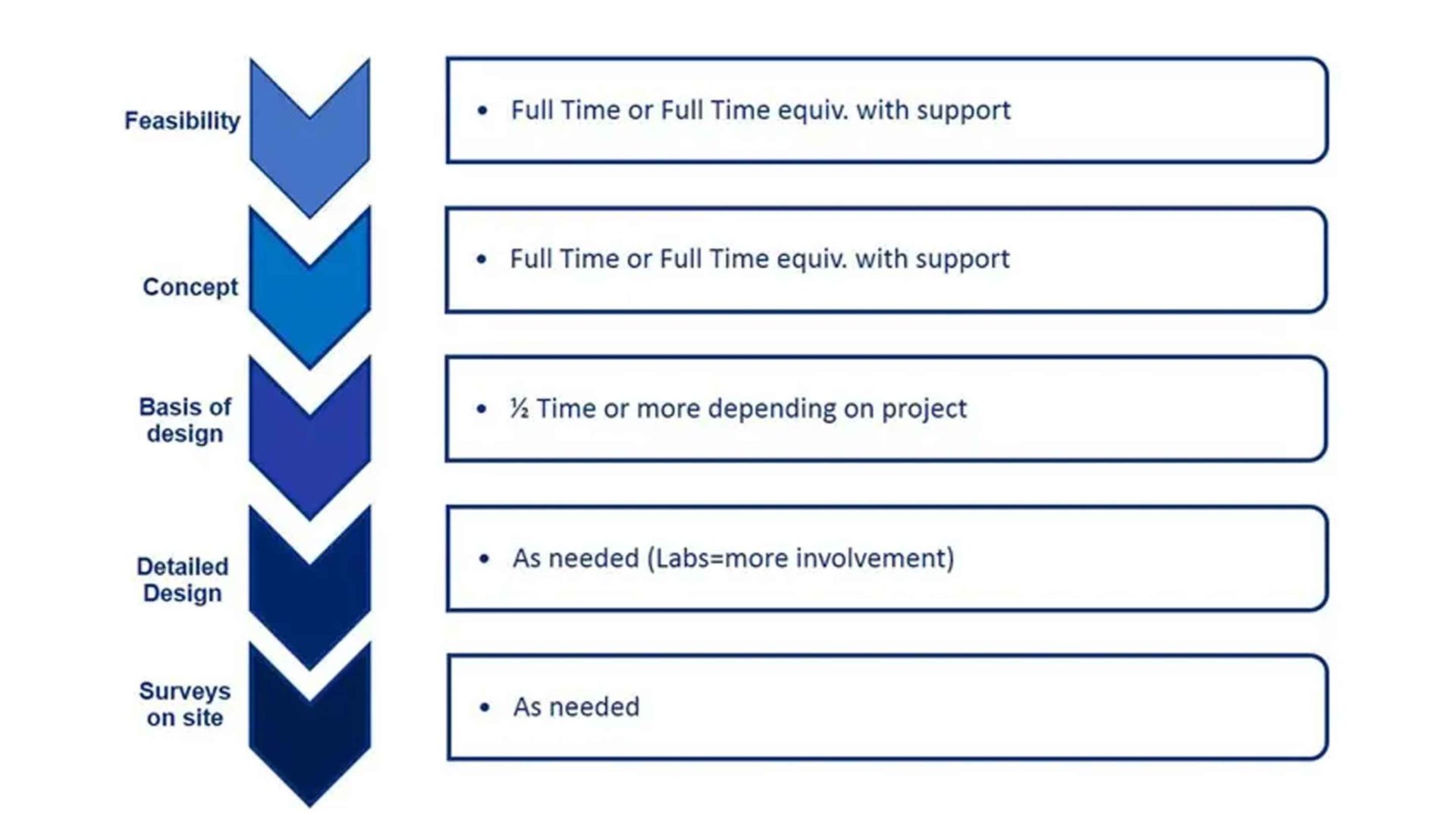
Process Architect involvement for project phases
In a project organization chart, it is usually the Project Engineer, who interfaces directly with the technical disciplines, responding to the PM for the correct flow of project information.
The Process Architect, on the other hand, has a role in defining the Layout, especially in the initial phases, interfacing in a privileged manner with the Client and with all the disciplines, guiding the coordination of the project team, in particular with the Process discipline, for the spatial coordination of structures, equipment and systems (Space Management).
Responsibilities and knowledge of the Process Architect
The Process Architect will be the figure who will guarantee an integrated inclusion of all these elements in the design, and not only that, but he will also aim for synergy between the systems; therefore, it is important that the Process Architect has technical skills so that he can define and develop:
Equipment Layout
- Project purpose
- Develop the adjacency diagram (hand drawings, sketchups, etc.)
- Sizing spaces and machines (GMP classes, flows, etc.)
- Know the project areas
- Apply Focus principles on sustainability
Flows
- Classification of spaces
- CCS (Contamination Control Strategy)
- Airlock requirements / strategy Gowning philosophy
- Material Flows
- Personal Flows
- Equipment
Finishes
- Integration of finishes
- Use of glass partitions
- Walkable false ceilings
- Finishes Workshops and case studies
- Chemical Resistance / Cleaning Chemicals Specifications
Coordination with technical disciplines
- Process
- HVAC
- Electrical
- Piping
- Automation, data, security
Development of maximum personnel requirements
- Lockers
- Offices
- Canteen
- Parking
IT tools
- Sketch Up
- Revit
- Corel or Adobe Illustrator/In-design
- Equipment library in Revit e Sketch Up
For the definition of the design elements mentioned above, 3D BIM, for example, even from the early design stages, becomes an excellent tool capable of exploiting the power of three-dimensional thinking and verifying the interference between the various project components.
Final considerations
As regards some final considerations on the figure of the Process Architect, it is worth focusing on the current state of the profession and on future market trends in the field of industrial production.
Evolution in the life sciences industry is driving the need for new manufacturing paradigms based on advanced technologies, and as a result process architects must transform themselves into systems integrators to better meet customer needs for fast manufacturing facilities.
For example, the constant introduction of new and innovative modular manufacturing solutions is creating significant opportunities in terms of cost and time of future production facilities.
A modern pharmaceutical production plant must be flexible and adaptable, not only to have the ability to scale up inventories to meet growing market demands, but also to be able to accommodate changes in production machinery needs.
Today, Process Architects are expanding their role as systems engineers and integrators focused on finding the best way to execute a project using modular and pre-engineered systems, through the design of the structure with the clear objective of improving quality, reducing at the same time the costs and time needed to deliver a project.
Process Architects are therefore among the key players in this team of designers who bring value to pharmaceutical projects.
Thanks to this know-how, Process Architects can act as true expert coordinators.
Articoli correlati
June 19, 2025
Values in Action: One Team, One Vision, One Culture
That’s why we dedicated time and space to a special moment of sharing and…
March 25, 2025
Sterilization Processes
The goal of any sterilization process is to destroy microorganisms present on a…
March 24, 2025
Commissioning of Plants: Importance, Process, and Benefits
The commissioning of plants is a crucial process to ensure the efficiency,…